Creating Concepts
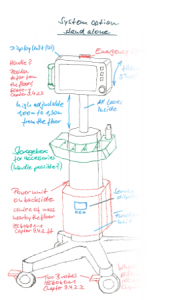
You provide the idea; we will check whether it can be realized. During the concept phase we can systematically support your creativity. Your product specifications will form the basis of our work.
We will also ensure that the final product adheres to legal and normative standards, as well as being suitable for manufacture, even in this phase. This means that we can minimize development risks, subsequent delays and cost increases early on in the process.
Requirement Management
You know best what you want your product to accomplish. You know your customers and know exactly what is expected of your products. But are you also aware which legal requirements and norms your product has to meet?
From your product requirements we will work out all the details, up to sub-system specifications and exact descriptions of individual parts in the plans. The usage of tools such as Caliber and DOORS ensures that you can track these specifications on the finished plan.
Construction
We have a lot of practical experience and thus know exactly what a construction has to look like in order to make the product safe, give it a high-quality finish and enable economical production.
Especially the specific requirements for medical devices (EN ISO 60601-1;EN ISO 13485; ISO 10993 etc.) play a vital role right from the beginning of construction.
We have a wealth of experience in working with SolidWorks, a CAD-tool which we have been using since the 98 version.
Through regular participation in professional training we ensure that innovations in CAD reach you directly, in order to make your constructions better and more efficient. New in August 2016: CATIA V5/V6.
Simulation
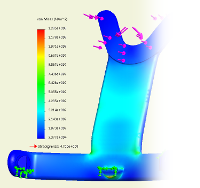
An FEM analysis of the construction helps us to eliminate mistakes early on in the development process.
All parts and sub-assemblies are optimized immediately with regard to manufacturing requirements and safety factors according to EN ISO 60601-1.
Unnecessary delays through later adjustments are avoided; negative test results can for the most part be precluded.
Material efficiency can immediately be improved, while the costs for development are kept to a minimum.
Prototyping
A plethora of functional prototypes, models of individual parts and samples for the validation of usability by your customers can be manufactured in our on-site workshop. Experience, craftsmanship and a focus on the demands of customers in the biomedical engineering sector guarantee a speedy and efficient manufacture.
For larger quantities and special orders we have access to a large network of qualified suppliers with the most recent technology.
Vendor Selection

Creating the relevant paperwork for a call for tenders, carefully examining offers and if necessary providing support in the qualification of suppliers can be crucial for the success of a construction.
We will take care that the data has no unwanted room for interpretation and that the demands regarding quality, adherence to delivery dates, and costs can be met.
No matter if a custom-made article or a larger series of products, a qualified supplier is vital for the perfect product.
Verification
While developing a new product one of the central questions is always: Will our idea work out as planned?
These questions have to be answered already in the early phases of development.
We can support you in developing engineering tests and providing the necessary equipment to run relevant tests.
Of course this support also extends to carrying out and evaluating these tests.
For the authorization of a medical device you will furthermore need proof of its regulatory demanded functions. We will develop test scenarios for these requirements and perform the tests in cooperation with accredited partners.
Naturally, we will produce appropriate documentation of the test results.
Fabrication Transfer
To enable the manufacture of a medical device in consistently high quality, the support of the departments for development is of great value. But also the early inclusion of the manufacture department into the process of development ensures that the practical knowledge of the manufacturers becomes part of the process. This is how unnecessary and often very costly detours can be avoided in the realization phase. Be it the construction of a production FMEA, the quality control of individual steps, the development of manufacturing tools or test systems; we establish ties between development and manufacture.